Scientists discover new optimism in fight against Alzheimer’s

Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.

This story is part of the Financial Times Seasonal Appeal for Alzheimer’s Research UK, which is raising funds for pioneering research to develop new treatments for dementia. You can support the appeal here
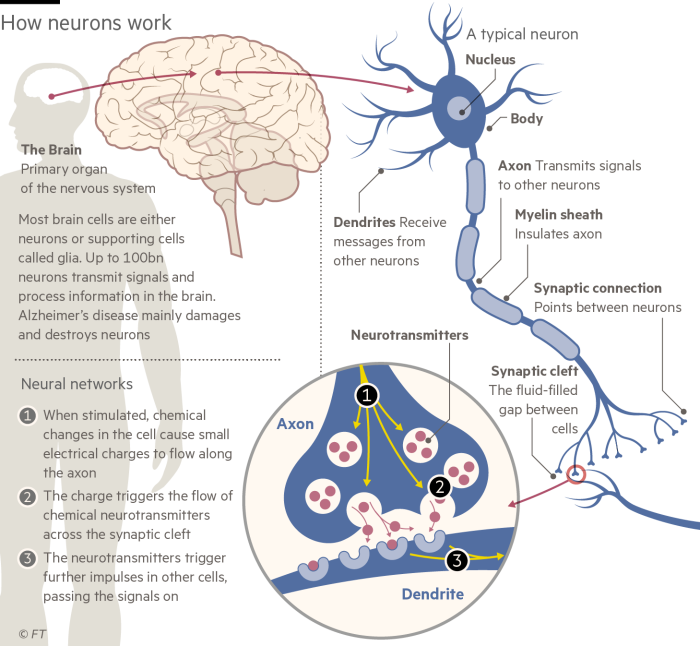
Professor Rick Livesey opens an incubator in his Cambridge lab and gets out a small plastic plate with six wells full of a faintly pink liquid. Each well contains a culture of stem cells recently created from the skin of a patient with Alzheimer’s disease.
Next he extracts a similar plate that has been maturing for 70 days at 37C. The stem cells have been converted into neurons, which we can see clumping together into patches of brain tissue.
A third plate contains specialist cells called microglia, which form the front line of the brain’s immune defences — and which are believed to play a key role in Alzheimer’s disease. We examine the strange tentacle-shaped microglia under a microscope, streaming towards a speck of fibre that has contaminated the culture.
“Microglia are clearly attracted to damage,” Prof Livesey says, “but, in Alzheimer’s, do they overreact or under-react to damage in the brain?”
To help answer that question and many others, his lab in Cambridge university’s Gurdon Institute has made multiple cell cultures from 100 people with inherited Alzheimer’s disease. Observing these cells’ biochemistry and behaviour, the scientists soon see clear differences between them and healthy neurons — giving clues to the fundamental processes involved in dementia.
This project is part of a worldwide flowering of academic and corporate research into Alzheimer’s and related dementias, as neuroscientists rally from disappointment over the pharmaceutical industry’s failure to develop a treatment that can even slow the development of disease. New routes are opening up potentially to prevent or reverse what is today seen as an inexorable downward course into dementia.
The philanthropist Bill Gates said this month that advances in neuroscience had convinced him to invest around $150m in dementia research. “I think we can develop an intervention that drastically reduces the impact of Alzheimer’s,” he tells the Financial Times. “There are plenty of reasons to be optimistic about our chances. Our understanding of the brain and the disease is advancing a great deal.”
As Mr Gates points out, Alzheimer’s is the only cause of death in the world’s top 10 that is becoming more prevalent over time — through demographic changes as the global population ages and the lack of “any meaningful treatments”.
According to Alzheimer’s Disease International, the global federation of Alzheimer’s societies, the number of people with dementia is now close to 50m. The total is projected to reach 75m in 2030. About 60 per cent of dementia cases are Alzheimer’s. The remaining 40 per cent are brain diseases with different biochemical and physiological features, which also cause symptoms of progressive memory loss, confusion and personality change; they include vascular dementia, Lewy body dementia and frontotemporal dementia.
ADI expects the financial burden of dementia to rise above $1tn in 2018 — more than 1 per cent of global gross domestic product — including costs attributed to unpaid care provided by family members, as well as direct costs of social care and medical care by health professionals.
Ever since Dr Alois Alzheimer identified the disease in 1906, scientists have been building up their knowledge of the distinctive anatomical changes that take place as the brain shrinks and symptoms develop. Over the past 30 years molecular and genetic research has pinpointed some of the biological processes involved, notably the build-up of toxic proteins that poison neurons. However, as Prof Livesey says, “we don’t know the fundamental cause of Alzheimer’s disease. We are not even close.”
The best known biological process — and the main target of drug development so far — is the amyloid pathway. This starts with amyloid precursor protein, which is present in all healthy cells (though scientists still do not know the function of APP). In diseased brains enzymes cut APP into protein fragments called amyloid beta, or aBeta, which then accumulate into the sticky insoluble “amyloid plaques” characteristic of Alzheimer’s.
A second disease process related to the amyloid pathway is the growth of “tau tangles” — insoluble twisted fibres inside the neurons. They are a misshapen form of a protein called tau that builds channels in healthy nerve cells.

Until very recently, Alzheimer’s drug development concentrated almost entirely on amyloid. Unfortunately, medicines designed to clear plaques from the brain have consistently failed to show benefits in clinical trials, leading many researchers to call for a change in direction.
“Our failing in the Alzheimer’s field is to have acted like a herd,” says Simon Lovestone, professor of translational neuroscience at Oxford university. “The argument that amyloid is important in Alzheimer’s is powerful but we have made a mistake over the past 20 years in focusing so much on amyloid.”
While scientists are pursuing many new approaches — looking, for example, at the role of the immune system and inflammation in the brain — amyloid retains a strong hold over Alzheimer’s research.

John Hardy, neuroscience professor at University College London and a discoverer of the amyloid pathway, insists that this is a primary cause of the disease rather than a secondary effect. Recent research shows that deposits of abnormal amyloid begin to build up in the brain 20-30 years before any symptoms appear, he says, followed later by tau protein.
“We failed to think enough about the length of the process, because lab experiments are relatively short term,” Prof Hardy adds. “Therapies have been given too late in the process, when amyloid is already maxed out. Now we are trying to get them into patients much earlier, though we do need also to add some other strings to our bow.”
Researchers have not given up on the amyloid attack. Clinical trials of amyloid-fighting drugs, including ones that failed in later-stage Alzheimer’s patients such as Eli Lilly’s solanezumab and Roche’s gantenerumab, are under way around the world in younger people who are at high risk because of their family history or genetic susceptibility.
Several experimental medicines are being studied at the National Hospital for Neurology and Neurosurgery in London. “Our subjects have no symptoms but evidence [from positron emission tomography scans] of amyloid in the brain,” says Dr Catherine Mummery, principal investigator. “They are in their 30s and 40s, so are working full-time.”
“There is a major ethical issue with asymptomatic subjects,” she adds. “Having to tell people that there is amyloid in their brain is an interesting discussion. We have to be very careful about the way we select individuals to take part.”

The biotech industry continues to develop more selective drugs aimed at specific targets within the amyloid pathway. Probiodrug, based in Germany, has identified an enzyme involved in the modification of aBeta into an especially harmful form — and is trialling drugs that block this particular step. ProMIS Neurosciences of Toronto is another company with drug candidates that aim to stop aBeta turning toxic.
The tau pathway is also attracting attention. AC Immune, a Swiss neuroscience company, is working on a three-pronged campaign against toxic tau tangles: an antibody, a small molecule and a vaccine designed to stimulate a patient’s own immune system to fight tau.
“We want to have an anti-tau and an anti-amyloid vaccine that you can give to a 40-year-old to prevent the onset of disease,” says Andrea Pfeifer, AC Immune chief executive. “Eventually clinicians will be able to tailor-make vaccines to treat each patient in a personalised way.”
Epitomising the new approaches to corporate Alzheimer’s research is the London-based Dementia Discovery Fund, in which Mr Gates has just invested $50m. DDF is an unusual commercial partnership launched two years ago to provide venture finance for innovative treatments; partners include the UK government, seven international drug companies and the charity Alzheimer’s Research UK — the beneficiary of this year’s FT Seasonal Appeal.
“The DDF will focus on new biology and won’t explore old technologies which have not yielded results,” says Kate Bingham of SV Health, the venture capital firm running DDF. Of the 12 start-ups and projects supported so far, none concentrates on amyloid and just one on tau.
She sees immuno-neurology — harnessing the power of the immune system to prevent neural disintegration — as a powerful weapon against dementia, rather as immuno-oncology is transforming cancer treatment. DDF’s first investment, California-based Alector, last month announced a $225m deal with AbbVie, the US pharmaceutical group, to develop immunotherapies for dementia.

Several lines of research, from genetic analysis to studies of animal models and human neurons, suggest that an important contributor to dementia is a failure of the brain’s immune system to clear away toxic and misshapen proteins. But how to enhance it is not clear.
Two main biological clearance systems remove undesirable material from the brain. One, discovered recently, is a series of channels known as the glymphatic system. Microglia, the brain’s specialist immune cells, are the other.
“You can think of the glymphatic system as drainage canals, while microglia are more like binmen actively removing rubbish,” says David Reynolds, chief scientist at Alzheimer’s Research UK. “The problem with the brain’s immune system is that it is hard to tell whether it is underactive or overactive.”
Jim Sullivan, head of pharmaceuticals discovery at AbbVie, goes for underactivity. “If you image amyloid plaques, you see them surrounded by microglia but they are not disappearing,” he says. “This suggests that the immune cells are not doing their job. Approaches to activate the glial cells and stop growth of the plaques could be very attractive.”
To accompany drug development, the field also needs good dementia diagnostics. “We are diagnosing the disease far too late,” says Dr Reynolds. “At the moment it usually happens through cognitive testing when you go to your doctor with thinking and memory problems. We know the disease will have started 20 years earlier.”
Although PET scans and analysis of cerebrospinal fluid can detect the build-up of amyloid before Alzheimer’s symptoms appear, the cost of these cumbersome procedures is slowing down clinical trials, says Prof Lovestone.

His lab is looking for biological markers of Alzheimer’s in blood — and a candidate, a protein called clusterin, has emerged. It would be combined with other indicator proteins in a blood test. “This would be used in clinical trials,” he says. “We are a long way from finding a screening test for use in the general population.”
Looking even further into the future, some experts see transplants of neural stem cells — like those made in Prof Livesey’s lab for studying Alzheimer’s — as a good prospect for replenishing the depleted brains of dementia patients.
“I think we will eventually get to the point where we can stop the progression of dementia if we start early enough,” says Dr Pfeifer of AC Immune. “Then we will need stem cell therapy to reverse the disease.”
Healthy lifestyle can stave off genetic susceptibility
The ultimate cause of Alzheimer’s disease remains mysterious, but we know that it results from a complex interaction between environment, genes and ageing.
The most important susceptibility gene in the general population is Apolipoprotein E, which is involved in processing lipids (fats) in the body. Someone who inherits a variant called ApoE4 from each parent is five times more likely than average to develop Alzheimer’s.
“The brain is a lipid-rich organ but we don’t know why ApoE has such a powerful effect on Alzheimer’s risk,” says John Hardy, a neuroscience professor at University College London. “About 30 genes are known to affect susceptibility to Alzheimer’s. In my lab we are working on predicting someone’s Alzheimer’s risk from their genes — and we can do that very well now.”
While there is nothing that can be done about genetic inheritance, lifestyles can be adapted to reduce the environmental risk. An expert commission set up by the Lancet reported in July that more than a third of dementia cases could be prevented by tackling non-genetic factors ranging from poor education, stress and obesity to hearing loss, head injury and pollution.
“There is a lot of research showing that healthy lifestyle can have a marked effect on your risks of developing dementia,” says David Reynolds, Alzheimer’s Research UK chief scientist. Advice that cuts the risk of other big killers such as cancer and heart disease is also helpful for fending off dementia.
Keeping the brain active may build up a protective “cognitive reserve”. This month a team based at the University of South Florida published results of a controlled trial showing that training the brain on specially designed computer games reduced the risk of dementia in people in their seventies and eighties. A regime called “speed of processing” training cut the risk by 29 per cent over 10 years, says Jerri Edwards, the study leader.
Rosa Sancho, ARUK head of research, is not getting excited. “The association between speed of processing training and lower dementia risk in the study is on the very edge of statistical significance, so we need to be cautious about drawing too firm a conclusion from this finding alone,” she says. “But remaining mentally active through life is an important part of a healthy lifestyle.”
• Your gift will be doubled
If you donate to Alzheimer’s Research UK through the FT’s Seasonal Appeal, Goldman Sachs has generously agreed to match it up to a total of £300,000. Click here to donate now
Comments